Accuracy and Precision Lab Chemistry Answers
Accuracy - An indication of how close a measurement is to the accepted value Precision - An indication of the degree of exactness of a measurement For multiple measurements. Mark each set of numbers as having a high or low accuracy and precision.
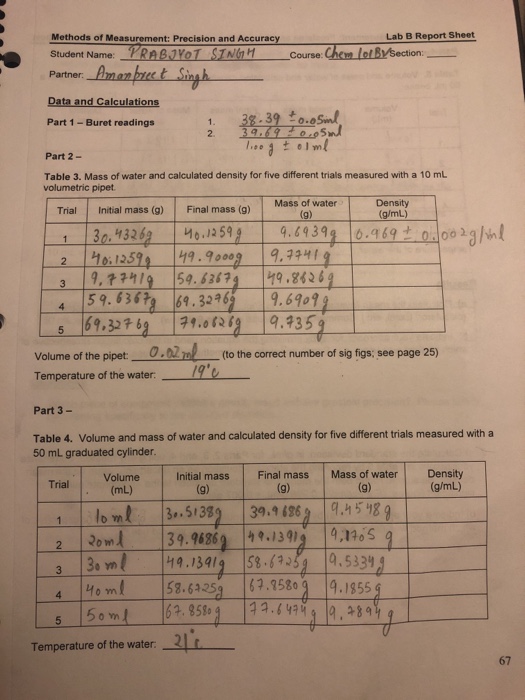
Solved Methods Of Measurement Precision And Accuracy Lab B Chegg Com
Delightful to help my own blog in this particular moment I will explain to you with regards to Accuracy And Precision Worksheet Answers.

. Unclear answers may result in loss of credit. In other words accuracy describes the difference between the measurement and the parts actual value while precision describes the variation you see when you measure the same part repeatedly with the same device. All measurements have some uncertainty associated with them.
Add about 10 mL of water to the 25 mL graduated cylinder. MCQ quiz on Accuracy and Precision multiple choice questions and answers on Accuracy and Precision MCQ questions quiz on Accuracy and Precision objectives questions with answer test pdf for interview preparations freshers jobs and competitive exams. 4 pts Replace the blue text below.
If you are told the length of. Written answers must be in complete sentences use proper grammar and have correct spelling. The accuracy of an experimental value is best determined by the average value of multiple measurements where x i represents a measurement and n is the number of measurements.
How experienced you are with using the device. What was the temperature of the DI water in the laboratory. Laboratory techniques of the analyst improve.
Accuracy and precision in chemistry do not mean the same thing and both are needed to have proper measurements. Measure and record the mass of the cylinder and water as accuratelyas possible. Accuracy is a measure of how close the measured value is to the true value.
Calculate the mass of the liquid for each trial. Since there are more than 2 sig figs in my answer I need to round it to the nearest tenths place. There is no such thing as a perfectly exact measurement.
Record this volume in the data table for part B. Subtract the mass of the empty graduated cylinder from the mass of the graduated cylinder with liquid Trial 1. Accuracy Precision and Error Lab Accuracy is the closeness of a measurement to the true value of what is being measured.
Accuracy and precision are both important to have in an experiment as it ensures both a correct result and reproducibility. Is actually that will awesome. 1 the precision in the measurements improves from the Devices C to B to A as indicated by the increasing trend in the SD.
Using a balance the most accurate glassware will deliver a mass of water closest to 1000 g and the most precise glassware will deliver a mass. Precision Device User The ability to reproduce a measurement consistently. His drives are precise but not accurate.
The variation observed when the same operator measures the same part repeatedly with the. Can cause uncertainty in experiments. If you believe consequently Il m demonstrate many graphic all over again down.
The level of accuracy required is usually. Precision but low accuracy. Laboratory apparatus and thinking about data analysis.
The student will be able to distinguish between the accuracy and precision of estimates made of the measure of several different quantities. When applied to scientific measurements the words accuracy and precision a. Add enough dry aluminum pellets so that the water level rises at least 5 mL.
What is the density of water at this temperature see table 1 in the experiment document. Lets assume the density of water at room temperature is 100 gmL. Study the definitions and comparisons of accuracy and precision in chemistry.
The answer is 43. Why dont you consider photograph previously mentioned. His drives are both accurate and precise.
Up to 24 cash back Chemistry. An analytical chemist carrying out measurements on a microscale eg weighing to 0001 mg may have to calibrate the balance each time they use it. Precision is the reproducibility of a set of measurements taken under the same conditions.
When rounding to the nearest tenths place I get the answer 44. Chemistry Name Date Class Accuracy and Precision Lab Accuracyhow close a measurement is to an accepted true value Precisiona tern used to describe how close repeated measurements are to each other Objective. In this lab exercise students practice correctly using measurement tools recording data calculating density using significant figures and exploring the concepts of accuracy and precision.
The least number of sig figs in the given measurements are 2 sig figs. For Devices C B A. Object measured is 10 meter long.
34 pts total Fill in the text boxes below. Accuracy How close a measurement is to some accepted true value. To assess accuracy you need to know the true or theoretical value from some source.
In this lab exercise students make measurements using common lab equipment and practice a wide range of calculations. Place 4 dots on each target with the appropriate level of accuracy and precision. 367584 4375 44.
Record the new water level in the table. Even airflow can upset very sensitive balances as can the temperature of the object being weighed. The trials have both accuracy and precisionthe densities were constantly with less than one point of value difference.
Precision can be broken down further into two components. Individual students was compared with data from the entire laboratory section. His drives are neither accurate nor precise.
Precision can also refer to how many digits are you able to measure with a piece of equipment sometimes referred to as numerical or arithmetic precision. The ability to read the proper decimal places from the device. Sure that they do give an accurate answer.
Accuracy but low precision. On the laboratory section data the most accurate piece of glassware was the PIPET and the most precise piece of glassware was the GRADUATED CYLINDER AND PIPET. Precision A term used to describe how close repeated measurements are to each other.
The precision of a set of measurements can be determined by calculating the standard deviation for a. Professionals Teachers Students and Kids Trivia Quizzes to test your knowledge on the subject. Be able to distinguish between the accuracy and precision of estimates made of the measure of several different quantities.
Accuracy and precision of scientific glassware. Have distinctly different meanings. Accuracy And Precision Worksheet Answers.
Create your account to access this entire worksheet. You are going to measure the mass and volume of several samples using different lab instruments then draw a graph and carry out density calculations.

The Purpose Of This Lab Was To Differentiate Between Accuracy And Precision By Calculating The Mass Per Millimeter Or The Density Of The Unknown Liquid Using Two Different Methods Gcse Science
Comments
Post a Comment